Abstract
Objectives
To assess the association between muscle invasion by oral squamous cell carcinoma of the posterior mandibular alveolar ridge and cervical lymph node metastasis on the basis of preoperative magnetic resonance imaging (MRI).
Materials and Methods
Twenty-six patients with oral squamous cell carcinoma of the posterior mandibular alveolar ridge were evaluated by MRI. The associations between cervical lymph node metastasis and independent factors evaluated by MRI were analyzed. Overall survival was also analyzed in this manner. Representative biopsy specimens were stained with anti-podoplanin and anti-CD34 antibodies.
Results
Mylohyoid muscle invasion was associated with cervical lymph node metastasis. A combinational factor of mylohyoid and/or buccinator muscle invasion was also associated with cervical lymph node metastasis. Cervical lymph node metastasis and masticator space invasion had a negative effect on overall survival. No lymphatic vessels were identified near the tumor invasion front within the mandible. In contrast, lymphatic vessels were identified near the front of tumor invasion in the muscles.
Cervical lymph node metastasis affects the prognosis of patients suffering oral squamous cell carcinoma (OSCC)12. Not only cervical metastasis with extranodular spread, but also occult metastasis reduce survival34. Therefore, physical examination, computed tomography (CT), ultrasonography, magnetic resonance imaging (MRI), positron emission tomography combined with CT, and sentinel node biopsy have been used to detect cervical lymph node metastasis from OSCC56789. However, undetected metastatic tumors might still be present as occult metastasis in some patients10.
The underlying mechanism of cervical lymph node metastasis from OSCC has been of interest. It is widely accepted that carcinoma cells undergo epithelial mesenchymal transition during the process of metastasis11. Epithelial mesenchymal transition of OSCC cells has been attributed to protein kinase B and transforming growth factor beta signaling, which activate transcription factors such as zinc finger protein SNAI1 1213. Cells in this transition are known to gain phenotypes that facilitate invasion and migration.
However, the anatomical factors that contribute to the metastatic process have not been fully evaluated. Typically, a significant proportion of OSCC of the posterior mandibular alveolar ridge tends to invade the mandible in its early course of disease owing to its proximity to the bone and is staged as T4a on the basis of bone invasion in the current American Joint Committee on Cancer cancer staging guidelines. Although the extent of mandibular invasion, evaluated by CT images, has been reported to be correlated with lymph node metastasis, evidence that bony invasion directly causes cervical lymph node metastasis is lacking14.
OSCC of the posterior mandibular alveolar ridge can invade nearby muscles or soft tissue spaces. The mylohyoid muscle forms the floor of the mouth adjacent to the mandible, and the buccinator muscle constitutes the buccal cheek. The sublingual space lies over the mylohyoid muscle, while the masticator space is positioned posteriorly. As far as the authors are aware, there has been no correlative study associating invasion into the aforementioned tissues with cervical lymph node metastasis. MRI and its superior soft tissue details have allowed detection of such invasions. An association between OSCC invasion into soft tissue and cervical lymph node metastasis will carry diagnostic implications.
In this report, we used preoperative MRI to correlate the status of probable anatomic etiologic factors with cervical lymph node metastasis in OSCC of the posterior mandibular alveolar ridge. Additionally, we examined the presence of lymphatic vessels in the mandible and attached muscles using immunohistochemical methods.
This study reviewed 113 patients who underwent mandibular resection surgery for ablation of OSCC in the Department of Oral and Maxillofacial Surgery, Seoul National University Dental Hospital (Seoul, Korea), during the period of January 2001 to March 2007. Patients with a history of neoadjuvant therapy were excluded from the study. Patients were further refined by excluding those with lesions located in any region other than the mandibular molar region. Finally, 26 patients with previously untreated squamous cell carcinoma of the posterior mandibular alveolar ridge were analyzed. Of these patients, 17 were male, and the average age at diagnosis was 64 years. The diagnoses were confirmed by histopathologic examination. The follow-up period ranged from 6 to 160 months, with an average of 69 months.
Twenty-four patients underwent neck dissection, and presence of cervical lymph node metastasis was determined by histopathologic examination of the neck specimen. Two patients with untreated neck were followed-up for at least 91 months with no evidence of cervical lymph node metastasis.
All patients underwent preoperative MRI before surgery. As we speculated that anatomical factors of the primary tumor would influence cervical lymph node metastasis, we assessed factors such as greatest tumor dimension, bone marrow invasion, sublingual space invasion, masticator space invasion, buccinator muscle invasion, and mylohyoid muscle invasion. Invasion of the bone marrow was assessed by determining the presence of tumor signals in the marrow space, in continuum to the primary tumor.(Fig. 1. A) Invasions of the sublingual space and masticator space were evaluated similarly.(Fig. 1. B, 1. C) Both buccinator muscle invasion and mylohyoid muscle invasion were determined by the integrity of the representative muscles and the presence of adjacent tumor.(Fig. 1. B, 1. D) An additional factor of muscle invasion was determined by presence of tumor invasion in at least one of the aforementioned muscles. The correlation of the factors assessed on MRI, as well as age and sex, with cervical lymph node metastasis was assessed by Fisher's exact test with a significance level of 0.05. SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Overall survival was also analyzed in relation to the forenamed factors as well as cervical lymph node metastasis. Survival time was determined as the interval in months between the date of surgery and death or the final follow-up visit for censored cases. A log-rank test was conducted to compare survival distribution according to presence or absence of each factor.
To verify the presence of lymphatic vessels at the tumor margin, immunohistochemistry was performed with tissue samples obtained from the study group. Representative specimens were obtained based on the availability of the tissue sample and the ability to identify the orientation of the tumor and the adjacent anatomical structures. Paraffin sections were cut (4 µm), deparaffinized, mounted on glass slides, and rehydrated. To identify the presence of lymphatic vessels, the rehydrated tissue sections were incubated with monoclonal anti-podoplanin antibody (1:100; AngioBio, San Diego, CA, USA) for 15 minutes. To confirm the identity of the capillary structures, complementary staining was performed with monoclonal anti-CD34 antibody (1:300; Immunotech, Prague, Czech Republic). Bound primary antibodies were visualized with the Bond Polymer Refine Detection kit (Leica, Wetzlar, Germany). Samples were observed and recorded using an optical microscope (Olympus, Tokyo, Japan) equipped with a charge-coupled device camera.
The associations between the assessed factors and cervical lymph node metastasis are shown in Table 1. Mylohyoid muscle invasion was associated with cervical lymph node metastasis. When mylohyoid muscle invasion and/or buccinators muscle invasion were combined as a single factor, the combinational factor of muscle invasion was also associated with cervical lymph node metastasis. No other variable was related to cervical lymph node metastasis.
The results of the log-rank test are shown in Table 2. Cervical lymph node metastasis had a negative effect on overall survival. Additionally, masticator space invasion was associated with reduced survival. No other variable had a significant impact on survival.
Podoplanin is a lymphatic endothelial cell-specific marker, while CD34 is expressed in blood vessel endothelial cells15. In the bone marrow, no lymphatic vessels were identified within or near the tumor by anti-podoplanin antibody staining.(Fig. 2. A) However, podoplanin-expressing endothelial cells were present in the submucosal connective tissue.(Fig. 2. B) Tubular structures present in the bone marrow were stained by anti-CD34 antibody, demonstrating its vascularity.(Fig. 2. C, 2. D) Lymphatic vessels were identified in muscles attached to the mandible and near the tumor invasion fronts within these muscles.(Fig. 2. E, 2. F)
Several factors are known to be related to cervical lymph node metastasis. Depth of invasion, tumor thickness, tumor consistency, and histologic differentiation of the tumor have been correlated with cervical lymph node metastasis in squamous cell carcinoma of the tongue16171819. In regard to carcinoma of the mandibular gingiva, advanced T stage, histologic or radiographic evidence of mandible invasion, and degree of tumor differentiation have been identified as predictive factors of cervical metastasis20. Ogura et al.1421 have reported that extent of both maxillary and mandibular bone invasion was predictive of cervical lymph node metastasis in gingival cancers.
Our results demonstrate that muscle invasion by squamous cell carcinoma of the posterior mandibular alveolar ridge is associated with cervical lymph node metastasis. Bone marrow invasion was not significantly associated with cervical lymph node metastasis. Although muscle invasion did not significantly affect survival, there was a tendency for better survival in patients without muscle invasion. Furthermore, the results of immunohistochemistry are concordant with the aforementioned results of our study and the findings of Edwards et al.22, which depict the absence of lymphatic vessels in normal cortical or cancellous bone. Intuitively, tumor portions lacking intra-tumoral or peri-tumoral lymphatic vessels are not suspect for cervical lymph node metastasis.
One possible explanation for our results is that muscle contracture could facilitate lymphatic drainage of cancer cells. High incidence of cervical lymph node metastasis from early OSCCs of the oral tongue or floor of the mouth23 supports this concept as these locations are the mobile subsites of the oral cavity. In contrast, tumors overlying the mandible might be shielded from distortion caused by muscle contracture.
It is noteworthy that bone marrow invasion was significantly associated with muscle invasion (P=0.001). This association implies that bone marrow invasion is a confounder in the association between muscle invasion and cervical lymph node metastasis. Previous reports that correlate the extent or pattern of mandible invasion to cervical lymph node metastasis can be interpreted in this context1424.
The diagnostic value of muscle invasion for predicting cervical lymph node metastasis in patients with OSCC of the posterior mandibular alveolar ridge was limited. Our results show a negative predictive value of 100% (8/8). However, the positive predictive value was 50% (9/18). Thus, presence of muscle invasion alone might not be sufficient for determining elective neck dissection. The survival benefit of elective neck dissection in patients with muscle invasion remains to be elucidated. The high negative predictive value of muscle invasion for diagnosis of cervical lymph node metastasis also requires further validation by large-scale, prospective studies.
References
1. Choi KK, Kim MJ, Yun PY, Lee JH, Moon HS, Lee TR, et al. Independent prognostic factors of 861 cases of oral squamous cell carcinoma in Korean adults. Oral Oncol. 2006; 42:208–217. PMID: 16249114.

2. Geum DH, Roh YC, Yoon SY, Kim HG, Lee JH, Song JM, et al. The impact factors on 5-year survival rate in patients operated with oral cancer. J Korean Assoc Oral Maxillofac Surg. 2013; 39:207–216. PMID: 24471047.

3. Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003; 39:130–137. PMID: 12509965.

4. Hiratsuka H, Miyakawa A, Nakamori K, Kido Y, Sunakawa H, Kohama G. Multivariate analysis of occult lymph node metastasis as a prognostic indicator for patients with squamous cell carcinoma of the oral cavity. Cancer. 1997; 80:351–356. PMID: 9241067.

5. Merritt RM, Williams MF, James TH, Porubsky ES. Detection of cervical metastasis. A meta-analysis comparing computed tomography with physical examination. Arch Otolaryngol Head Neck Surg. 1997; 123:149–152. PMID: 9046281.

6. Krestan C, Herneth AM, Formanek M, Czerny C. Modern imaging lymph node staging of the head and neck region. Eur J Radiol. 2006; 58:360–366. PMID: 16687230.

7. Veit P, Ruehm S, Kuehl H, Stergar H, Mueller S, Bockisch A, et al. Lymph node staging with dual-modality PET/CT: enhancing the diagnostic accuracy in oncology. Eur J Radiol. 2006; 58:383–389. PMID: 16476533.

8. Civantos F, Zitsch R, Bared A. Sentinel node biopsy in oral squamous cell carcinoma. J Surg Oncol. 2007; 96:330–336. PMID: 17879335.

9. Yu MG, Ryu SY. Usefulness of 18F-FDG PET/CT in the evaluation of cervical lymph node metastasis in patients with oral cancer. J Korean Assoc Oral Maxillofac Surg. 2009; 35:213–220.
10. Kang JH, Ahn KM, Lee SW, Myoung H, Lee JH, Kim MJ. Neck dissection of clinically N0 Neck of oral squamous cell carcinoma & pathologic comparison. J Korean Assoc Oral Maxillofac Surg. 2007; 33:591–596.
11. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009; 119:1420–1428. PMID: 19487818.

12. Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, et al. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003; 63:2172–2178. PMID: 12727836.
13. Qiao B, Johnson NW, Gao J. Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-beta1 is Snail family-dependent and correlates with matrix metalloproteinase-2 and -9 expressions. Int J Oncol. 2010; 37:663–668. PMID: 20664935.

14. Ogura I, Kurabayashi T, Amagasa T, Okada N, Sasaki T. Mandibular bone invasion by gingival carcinoma on dental CT images as an indicator of cervical lymph node metastasis. Dentomaxillofac Radiol. 2002; 31:339–343. PMID: 12424630.

15. Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999; 154:385–394. PMID: 10027397.
16. Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997; 19:205–210. PMID: 9142520.

17. Asakage T, Yokose T, Mukai K, Tsugane S, Tsubono Y, Asai M, et al. Tumor thickness predicts cervical metastasis in patients with stage I/II carcinoma of the tongue. Cancer. 1998; 82:1443–1448. PMID: 9554518.

18. Ogura I, Amagasa T, Miyakura T. Correlation between tumor consistency and cervical metastasis in tongue carcinoma. Head Neck. 2000; 22:229–233. PMID: 10748445.

19. Kurokawa H, Yamashita Y, Takeda S, Zhang M, Fukuyama H, Takahashi T. Risk factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002; 24:731–736. PMID: 12203797.

20. Eicher SA, Overholt SM, el-Naggar AK, Byers RM, Weber RS. Lower gingival carcinoma. Clinical and pathologic determinants of regional metastases. Arch Otolaryngol Head Neck Surg. 1996; 122:634–638. PMID: 8639295.

21. Ogura I, Kurabayashi T, Sasaki T, Amagasa T, Okada N, Kaneda T. Maxillary bone invasion by gingival carcinoma as an indicator of cervical metastasis. Dentomaxillofac Radiol. 2003; 32:291–294. PMID: 14709602.

22. Edwards JR, Williams K, Kindblom LG, Meis-Kindblom JM, Hogendoorn PC, Hughes D, et al. Lymphatics and bone. Hum Pathol. 2008; 39:49–55. PMID: 17904616.

23. Ferlito A, Silver CE, Rinaldo A. Elective management of the neck in oral cavity squamous carcinoma: current concepts supported by prospective studies. Br J Oral Maxillofac Surg. 2009; 47:5–9. PMID: 19121878.

24. Brown JS, Lowe D, Kalavrezos N, D'Souza J, Magennis P, Woolgar J. Patterns of invasion and routes of tumor entry into the mandible by oral squamous cell carcinoma. Head Neck. 2002; 24:370–383. PMID: 11933179.

Fig. 1
Anatomical factors assessed on preoperative magnetic resonance imaging. Examples of bone marrow invasion (arrow) (A), sublingual space invasion (arrowhead) and buccinator muscle invasion (arrow) (B), masticator space invasion (arrow) (C), and mylohyoid muscle invasion (arrow) (D) are shown.
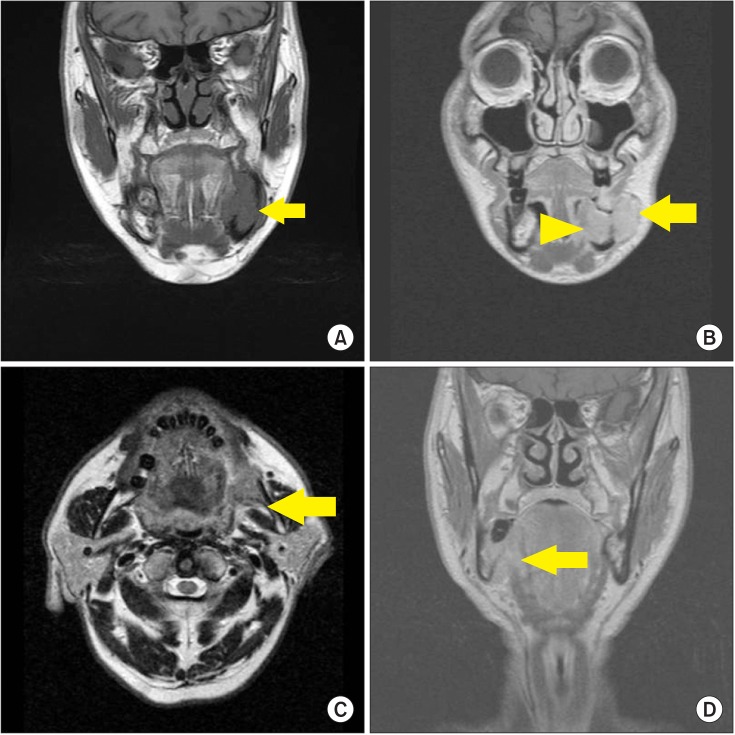
Fig. 2
Immunohistochemical stainings of sections from representative specimens are shown. No lymphatic vessels are identified in the tumor front within the marrow space of the mandible (A), while lymphatic vessels (arrows) are present at the submocosa overlying the mandible (B). Both Fig. 2. A and 2. B are different areas of the same section stained with anti-podoplanin antibody (A: ×100, B: ×200). With no evidence of lymphatic vessels near the bone marrow invasive tumor front in the anti-podoplanin-stained section (C) (×100), the capillary structures are variously stained by anti-CD34 antibody in a serial section (D) (×100). In contrast with the bone marrow, muscles attached to the mandible contain numerous lymphatic vessels (arrows) (E), thus when the tumor invades the muscles, they have close access to the lymphatic vessels (F). Fig. 2. E and 2. F stained with anti-podoplanin antibody (E: ×200, F: ×100).
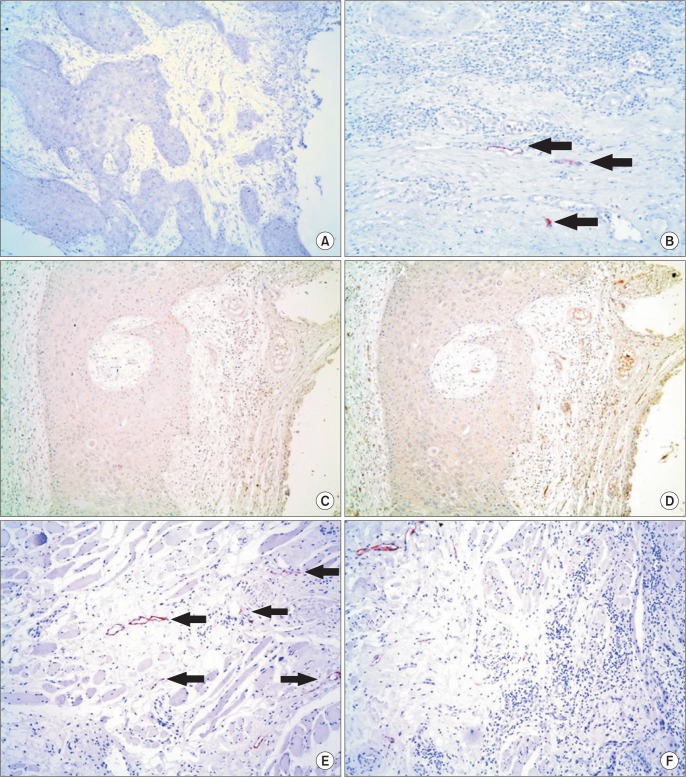
Table 1
Correlation of factors to cervical lymph node metastasis (CLNM)
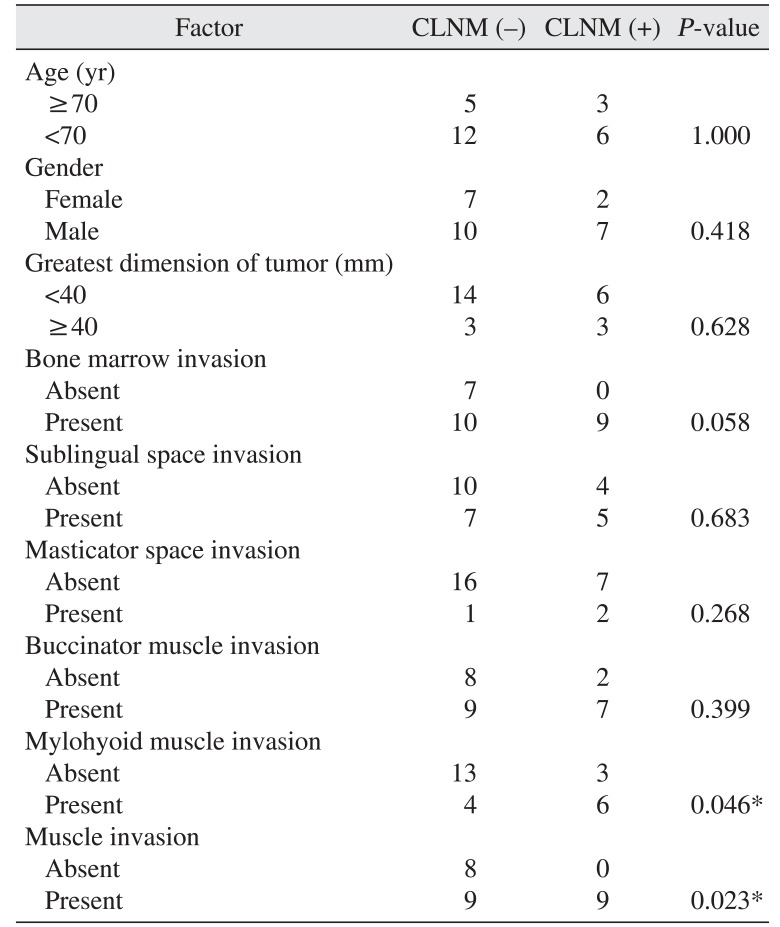
Table 2
Differences in overall survival distributions




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download